Rapid diagnostic tests are serological tests that are used in preliminary medical screening or incase of emergency diagnosis.
- They either detect antibody (Ab) or antigen (Ag) that an individual develops against the infection or the viral protein i.e, the antigen of the pathogen.
There are two types of rapid diagnostic test:
Why to use rapid diagnostic tests?
- Conventional microbiological tests usually take 2 to 7 days for an organism to be identified from sampling, inoculation, incubation to the identification, and susceptibility testing. Therefore, rapid diagnostic tests are easy to use and its test duration requires 10 minutes to 2 hours only.
- Rapid diagnostic tests are inexpensive and require less time and labor.
- Intensive training or professional workers to use the rapid test kit is not required as it has a simple procedure that can be followed easily.
Principle of RDTs
RDT is a qualitative and semi-quantitative – Vitro diagnostic medical device that works on the basis of immunochromatographic action like lateral flow or agglutination that forms the antigen-antibody complexes with the specific antigen of the pathogen from the given sample.
Dipstick, microfluidics, and cassette formats are often used with a sample on the test card along with certain reagents that provide a result within half an hour.
Types of rapid diagnostic tests
1. Rapid antibody test:
- A rapid antibody test kit detects the presence of a patient-generated antibody that is produced in response to a certain infection.
- Generally, IgG and IgM antibody isotypes are detected in this test.
- IgM is an initial antibody that is produced against the infection whereas the IgG antibody appears later during the infection.
- These antibodies are present in the blood sample when a person has an infection and gives a positive test result.
Procedure of Rapid Antibody Test
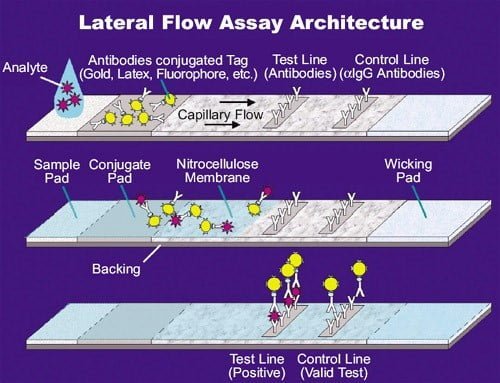
(Source: https://asm.org/Articles/2020/August/How-the-SARS-CoV-2-EUA-Antigen-Tests-Work )
- The sample (blood, plasma, or serum) is taken and added to the well of the test card.
- About 10 mM dilution buffered saline is also added.
- Due to the capillary action, the mixture flows down to the test pad.
- Once the sample hits the conjugation pad, the antibodies present in the sample binds to the antigen which is already present in the conjugation pad with conjugated gold nanoparticles.
- Then the formed conjugate complex passes to the nitrocellulose membrane and comes in contact with the three test lines: M, G, and control.
- M test line is for the recognition of the human IgM antibody and gives a visible colored line only if the body produces this antibody against a certain infection.
- G test line for the recognition of the human IgG antibody in response to an infection and produces a visible colored line when binds and forms IgG antibody/antigen/gold nanoparticle complexes.
- The control line remains at the end and a colored line should always appear in this region to make sure that the proper volume of the sample has been added.
- The excess sample will flow to the absorption pad.
- The results will be visible within 10 minutes.
2. Rapid antigen test
Rapid antigen kit detects the presence of specific viral antigen which indicates the current viral infection.
This test is commonly used for the detection of respiratory pathogens like the influenza virus, respiratory syncytial virus (RSV), and coronavirus.
Procedure of Rapid Antigen test

(Source: https://www.rxdx.in/rapid-antigen-test/ )
- Usually, nasopharyngeal or nasal swab specimens are collected as a sample.
- Samples should be collected only by professional healthcare workers and should be conducted within an hour of sample collection. But if the sample needs to be transported for long distances, a viral transport medium (VTM) must be used.
- Collected samples are directly placed into the assay’s extraction buffer or reagent.
- Squeezing the swab on the side of the tube is done so that all the liquid gets extracted on the tube.
- About 3 to 4 drops of the extracted sample is placed on the well of the test cassette.
- The result will be visible within an hour.
- A positive result gives a visible colored band and needs no further confirmation while a negative result gives no colored band and requires further RT – PCR test to confirm infection.
Reliability and Interpretation of test result
- The sensitivity of the test result is the ability to identify the patient with disease correctly. The sensitivity of most tests is >95%.
- The specificity of the test result is the ability to identify patients without disease correctly. The specificity of most tests is >99%.
- In a certain disease outbreak, these rapid diagnostic tests are done and for infected people, the test result will be positive and for not infected will be negative.
- But due to some circumstances, the test result will give false positive and false negative results that do not match the patient’s status.
- False-positive is when a healthy individual gets mistakenly identified as sick whereas false-negative indicates a sick patient incorrectly tests as healthy.
- False-positive results are more likely to occur when the prevalence of the viral disease is low in the community.
- False-negative results are more likely to occur when the prevalence of the viral disease is high in the community.
- To minimize these false results the test kits with high sensitivity and specificity must be used.
- Similarly, collecting samples within 3 to 4 days of illness and following the manufacturer’s instructions properly while testing helps in minimizing these consequences.
References:
- https://en.wikipedia.org/wiki/Rapid_diagnostic_test
- https://www.cdc.gov/flu/professionals/diagnosis/rapidclin.htm
- https://www.finddx.org/wp-content/uploads/2020/05/FIND_COVID-19_RDTs_18.05.2020.pdf
- https://www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/Generic_pf_training_manual_web.pdf?ua=1
- https://www.who.int/malaria/areas/diagnosis/rapid_diagnostic_tests/en/
- https://thebiologynotes.com/real-time-pcr-and-rapid-diagnostic-test/
- https://www.elisagenie.com/rapid-covid19-antibody-detection-tests-principles-and-methods
- https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html#table2